Page 76 - Science - 5
P. 76
w are arranged in any particular pattern.
w are loosely packed and keep moving.
Quenching Your Scientific Thirst
Quenching Your Scientific ThirstQuenching Your Scientific ThirstQuenching Your Scientific ThirstQuenching Your Scientific ThirstQuenching Your Scientific Thirst
Quenching Your Scientific ThirstQuenching Your Scientific ThirstQuenching Your Scientific ThirstQuenching Your Scientific ThirstQuenching Your Scientific Thirst
Quenching Your Scientific Thirst
The tiny particles that matter is composed of are actually called
molecules.
SOLUTIONS
A simple solution is basically
comprised of two substances that
are mixed together. One of them
is called the solute . A solute is the
substance to be dissolved (e.g. :
sugar). The other is a solvent . The
solvent is the substance in which
the solute dissolves (e.g. : water).
A solution is a homogeneous mixture in which the molecules of the solute are
distributed uniformly throughout the solvent. There is another type of mixture
called heterogenous mixture. In this type of mixture, there is no blending of
the substances. The substances of the mixture are distinctly visible.
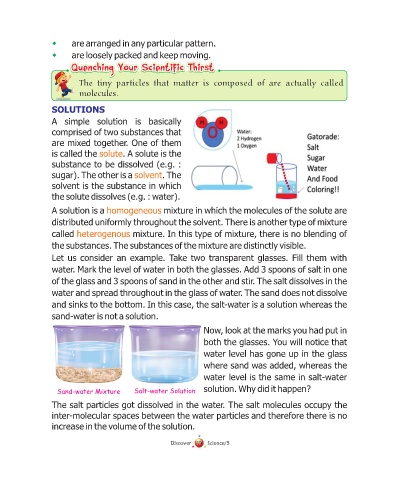
Let us consider an example. Take two transparent glasses. Fill them with
water. Mark the level of water in both the glasses. Add 3 spoons of salt in one
of the glass and 3 spoons of sand in the other and stir. The salt dissolves in the
water and spread throughout in the glass of water. The sand does not dissolve
and sinks to the bottom. In this case, the salt-water is a solution whereas the
sand-water is not a solution.
Now, look at the marks you had put in
both the glasses. You will notice that
water level has gone up in the glass
where sand was added, whereas the
water level is the same in salt-water
Sand-water Mixture Salt-water Solution solution. Why did it happen?
The salt particles got dissolved in the water. The salt molecules occupy the
inter-molecular spaces between the water particles and therefore there is no
increase in the volume of the solution.
Discover 77 Science/5
77
77